Efficacy and Safety of Omilancor in a Phase 2 Randomized, Double-blind, Placebo-controlled Trial of Patients with Ulcerative Colitis
Introduction. Omilancor is an oral, gut-restricted, small molecule, first-in-class LANCL2 agonist for ulcerative colitis (UC) and Crohn’s disease (CD). Through LANCL2 activation, omilancor locally increases the suppressive capacity of regulatory immune cells, including regulatory CD4+ T cells (Tregs), in the intestinal mucosa. Once daily omilancor was well tolerated up to doses of 7500 mg/day in healthy volunteers in Phase 1.
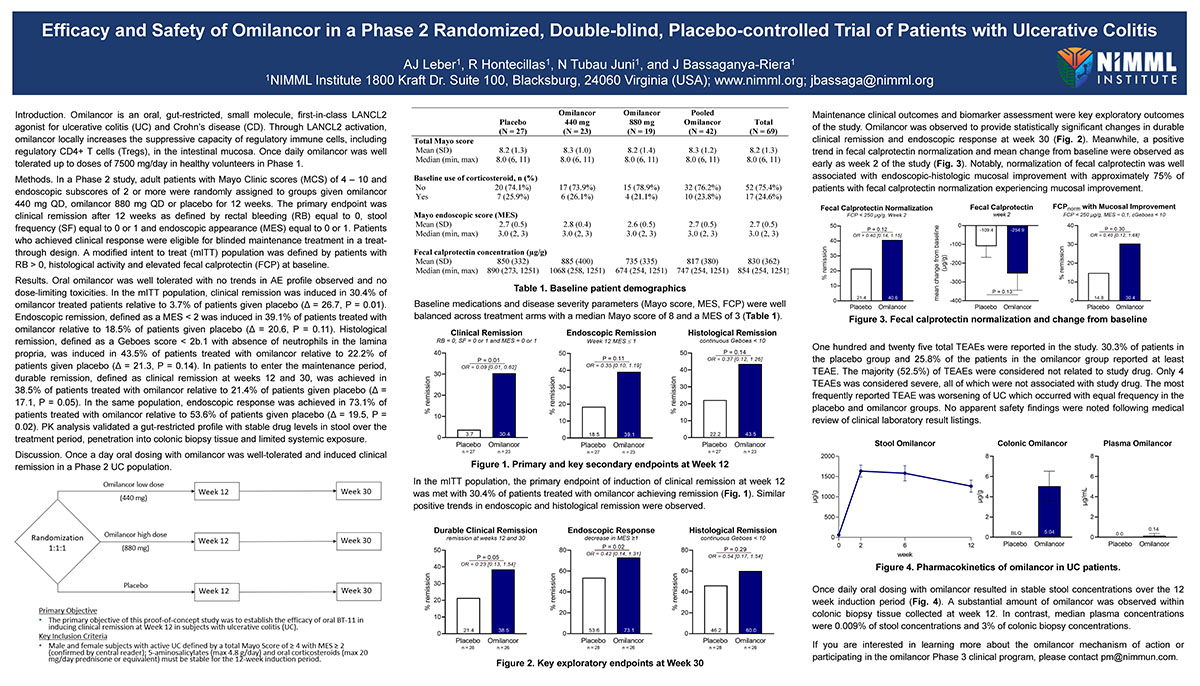
Methods. In a Phase 2 study, adult patients with Mayo Clinic scores (MCS) of 4 – 10 and endoscopic subscores of 2 or more were randomly assigned to groups given omilancor 440 mg QD, omilancor 880 mg QD or placebo for 12 weeks. The primary endpoint was clinical remission after 12 weeks as defined by rectal bleeding (RB) equal to 0, stool frequency (SF) equal to 0 or 1 and endoscopic appearance (MES) equal to 0 or 1. Patients who achieved clinical response were eligible for blinded maintenance treatment in a treat-through design. A modified intent to treat (mITT) population was defined by patients with RB > 0, histological activity and elevated fecal calprotectin (FCP) at baseline.
Results. Oral omilancor was well tolerated with no trends in AE profile observed and no dose-limiting toxicities. In the mITT population, clinical remission was induced in 30.4% of omilancor treated patients relative to 3.7% of patients given placebo (Δ = 26.7, P = 0.01). Endoscopic remission, defined as a MES < 2 was induced in 39.1% of patients treated with omilancor relative to 18.5% of patients given placebo (Δ = 20.6, P = 0.11). Histological remission, defined as a Geboes score < 2b.1 with absence of neutrophils in the lamina propria, was induced in 43.5% of patients treated with omilancor relative to 22.2% of patients given placebo (Δ = 21.3, P = 0.14). In patients to enter the maintenance period, durable remission, defined as clinical remission at weeks 12 and 30, was achieved in 38.5% of patients treated with omilancor relative to 21.4% of patients given placebo (Δ = 17.1, P = 0.05). In the same population, endoscopic response was achieved in 73.1% of patients treated with omilancor relative to 53.6% of patients given placebo (Δ = 19.5, P = 0.02). PK analysis validated a gut-restricted profile with stable drug levels in stool over the treatment period, penetration into colonic biopsy tissue and limited systemic exposure.
Discussion. Once a day oral dosing with omilancor was well-tolerated and induced clinical remission in a Phase 2 UC population.