Landos Biopharma Announces Final Safety Results from Phase 1 Study of BT-11 in Healthy Volunteers
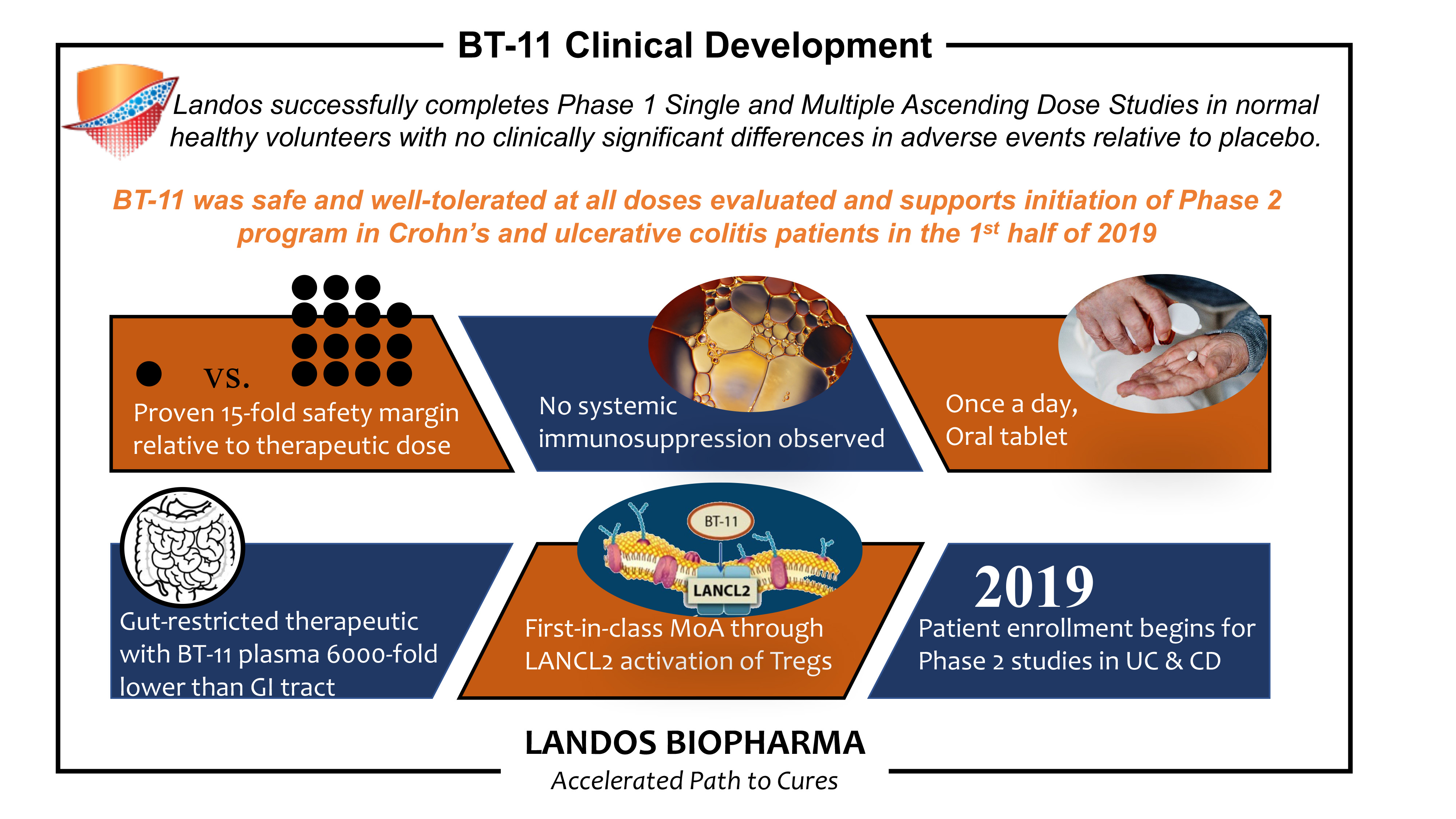
- BT-11 was safe and well-tolerated at all doses evaluated and supports initiation of Phase 2 program in IBD patients in 1st half of 2019 -
BLACKSBURG, VA, January 7, 2019 – Landos Biopharma, Inc., an emerging clinical-stage, biopharmaceutical company focused on developing improved treatments for autoimmune diseases, today announces final results from the Phase 1 study of BT-11, a first-in-class, orally active, gut-restricted therapeutic for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD).
The randomized, double-blind, placebo-controlled trial conducted in 70 healthy subjects to assess the safety of BT-11 found that BT-11 was well-tolerated and showed no dose-limiting toxicities. Among the subjects, the overall adverse events (AEs) profile observed was consistent when compared to AEs in the placebo cohort and no differences were observed between groups. The study tested levels of BT-11 ranging from 7 mg/kg up to 100 mg/kg and did not reach a maximum tolerated dose.
“The results of our Phase 1 study provide the first-in-human clinical data in support of BT-11 as a novel oral treatment for IBD. We are pleased to see that BT-11 was well-tolerated and safe in healthy volunteers,” said Dr. Josep Bassaganya-Riera, Chairman and CEO of Landos. “Importantly, we are very encouraged by the reduction of fecal calprotectin in BT-11-treated subjects, which could represent an early anti-inflammatory signal, and supports the potential of BT-11 as an important advancement in the treatment of inflammatory bowel disease. These study results, along with extensive preclinical efficacy models of IBD, support the evaluation of BT-11 in Phase 2 proof-of-concept efficacy studies in UC and CD patients, which we plan to initiate this year.”
“These results are promising for IBD patients in need of a more convenient treatment for UC and CD,” said Jean-Frederic Colombel, MD, a Landos Clinical Advisory Board member, world-renowned gastroenterologist and Director of the IBD Center at the Icahn School of Medicine at Mount Sinai. “We continue to see an unmet clinical need for chronic oral therapies to treat UC and CD that can improve safety, tolerability and convenience.”
About the Phase 1 Trial
This randomized, double-blind, placebo-controlled Phase 1 trial evaluated the safety and tolerability of BT-11 in five cohorts of single ascending and three cohorts of multiple ascending dose studies in 70 healthy subjects. The study tested levels of BT-11 ranging from 7 mg/kg up to 100 mg/kg. Within the subject group of the study, BT-11 was well-tolerated and showed no safety concerns or dose-limiting toxicities.
In addition, biomarker and immune screening results indicated that subjects in the single and multiple ascending dose studies had lower levels of fecal calprotectin, an inflammatory biomarker, compared to the placebo cohort. In addition, BT-11 did not induce systemic immunosuppression as measured by white blood cell counts relative to placebo in either study.
There were no dose dependent relationships in gastrointestinal-related AEs observed in both the single and multiple ascending dose studies. There was no significant difference in AEs between the dosed subjects and the placebo cohort.
About BT-11
Landos’ lead clinical asset, BT-11, is a novel, oral, gut-restricted, small molecule targeting the Lanthionine Synthetase C-Like 2 (LANCL2) pathway in the gastrointestinal tract for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). BT-11 has shown outstanding therapeutic efficacy in preclinical models of inflammatory bowel disease (IBD), a benign safety profile without the concerns of systemic exposure, and has two open Investigational New Drug (INDs) for evaluation in UC and CD. BT-11 intercepts IBD by decreasing the production of inflammatory mediators and increasing anti-inflammatory molecules within the gastrointestinal tract.
About IBD
IBD represents a group of chronic and disabling disorders that greatly impact a patient’s quality of life. The two primary clinical manifestations of IBD - Crohn’s disease (CD) and ulcerative colitis (UC) - afflict 2 million North Americans and 5 million people worldwide, with nearly 25% growth in prevalence over the last five years. There is an unmet clinical need for safer, more effective medications for these diseases as currently marketed therapeutics have a number of drawbacks: they only benefit a small number of the overall population, lose response effectiveness, or cause high rates of serious side effects, including cancer, infection, and death.
About Landos Biopharma
Landos Biopharma, Inc. is a clinical-stage biopharmaceutical company focused on the discovery and development of first-in-class oral therapeutics for patients with autoimmune diseases. Landos’ lead clinical asset, BT-11, is a first-in-class, oral therapeutic that acts locally in the gastrointestinal tract for treatment of inflammatory bowel disease (IBD). The company has completed Phase 1 clinical testing and plans to initiate Phase 2 clinical testing of BT-11 for inflammatory bowel disease in 2019. Landos also has a robust pipeline of new compounds for other autoimmune diseases, several of which will advance to IND in 2019. Landos is headquartered in Blacksburg, VA. For more information,please visit www.landosbiopharma.com or contact info@landosbiopharma.com or follow us @Landosbio.
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit public charity foundation focused on a transdisciplinary, team-science approach to precision medicine at the interface of immunology, inflammation, and metabolism. The NIMML Institute team has led numerous large-scale transdisciplinary projects and is dedicated to solving important societal problems by combining the expertise of immunologists, computational biologists, toxicologists, modelers, translational researchers, and molecular biologists. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
