The NIMML Institute Launches New Tool for Rapidly Analyzing Host Metabolic Profiles from Global Transcriptomics Datasets
The NIMML Institute Launches New Tool for Rapidly Analyzing Host Metabolic Profiles from Global Transcriptomics Datasets
The innovative M2 pipeline strengthens the advanced metabolic modeling functionality of NIMML’s TITAN-X precision medicine platform for drug development
The TITAN-X platform is an integrated computational modeling, bioinformatics and A.I. system designed to accelerate clinical development of precision medicines for human diseases
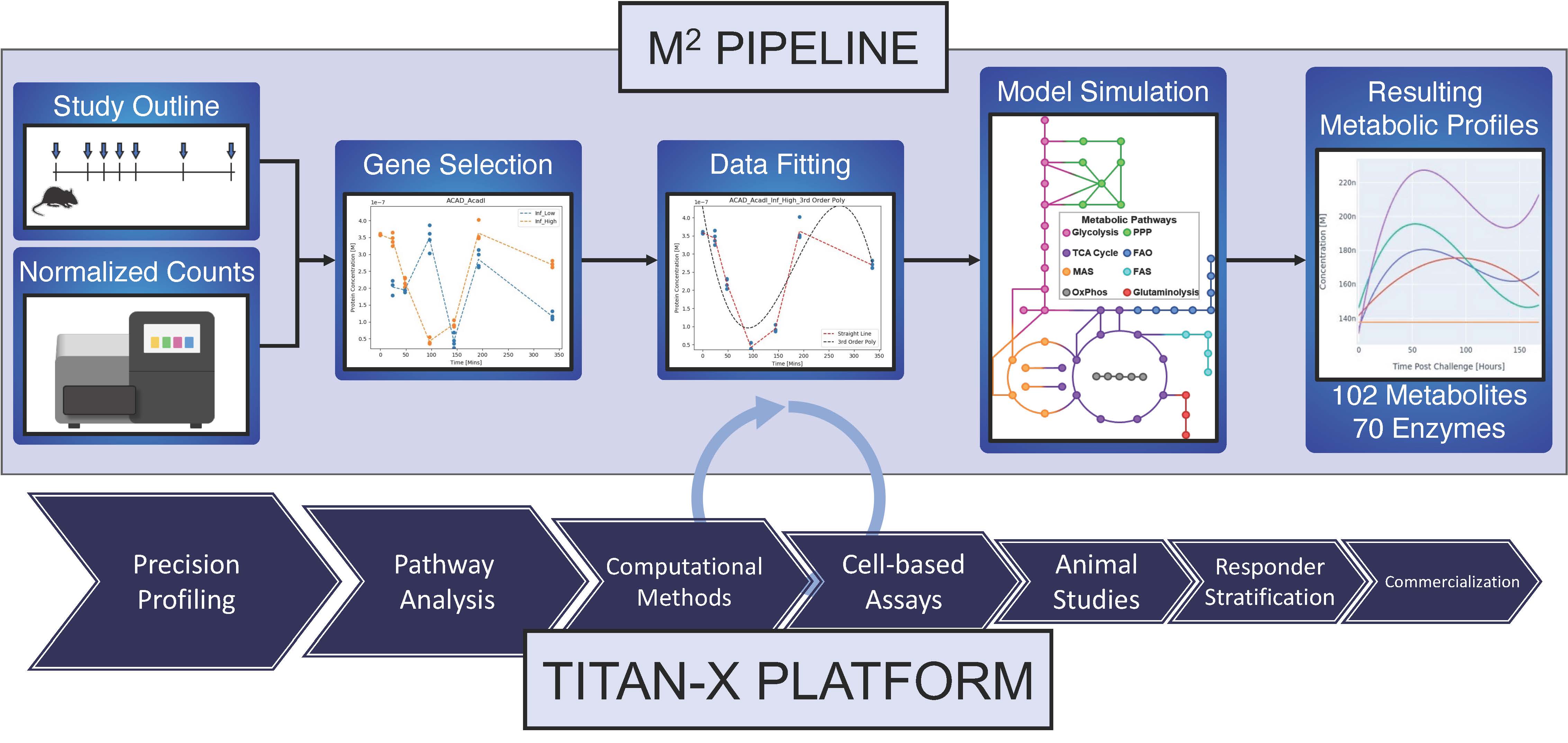
Blacksburg, VA., May 22, 2023 – The NIMML Institute (“NIMML”), a 501 (c) (3) non-profit foundation focused on applying transdisciplinary, team-science approaches to precision medicine, has unveiled a new groundbreaking bioinformatics and data analytics pipeline that can predict metabolic profiles from global gene expression datasets. The study, presented during the 11th International Conference on Bioinformatics and Computational Biology (ICBCB) and published in the ICBCB 2023 proceedings, describes how the Modeling Metabolism (M2) Pipeline was used to analyze and compare complex metabolic profiles of four viral respiratory infections: rhinovirus infection, flu, COVID-19, and MERS-CoV infection, based on host RNA-seq datasets. The M2 pipeline is the newest addition to NIMML’s TITAN-X Drug Discovery and Development Platform.
“Building on our experience leading one of four nationwide centers of modeling immunity for biodefense at the helm of the Modeling Immunity to Enteric Pathogens center, which created computational and mathematical models of the immune system of unprecedented scalability, the M2 pipeline represents the latest addition to the NIMML Institute’s unique arsenal of advanced computational capabilities for accelerating our drug development efforts.” said Dr. Josep Bassaganya-Riera, President and Founding Director of the NIMML Institute. “The M2 Pipeline is disease-agnostic and broadly applicable to study host responses to biological, chemical, or nuclear threats as well as accelerating drug development efforts.”
The M2 pipeline utilizes global gene expression datasets in combination with advanced computational modeling of metabolic pathways. By integrating theoretical and data-driven approaches, the M2 pipeline provides valuable new mechanistic insights into host-pathogen interactions and highlights metabolic changes that play important roles in the mechanisms of disease pathogenesis.
“With sixty-two key enzymes and almost one hundred metabolites represented in this ordinary differential equation metabolic model, the M2 pipeline can accurately predict changes in enzymatic activities and concentration of metabolites, plus production rates of ATP and other metabolic cofactors, during disease and tissue pathology. Importantly, it can be used to predict the unique metabolic fingerprint of a pathogen on its host, which could provide initial insights into pathogenesis and severity of that threat before a pathogen is even identified. Therefore, the M2 pipeline can be used to increase the level of public health preparedness by rapidly identifying and responding to the sudden emergence of previously unknown infectious diseases based on host response data.” said Dr. Dr. Hontecillas, CSO of the NIMML Institute.
The results of this study, which was funded by the Defense Threat Reduction Agency, indicated a distinctive host metabolic fingerprint associated with the severity of each infection. Influenza virus, SARS-CoV-2, and MERS-CoV infections resulted in increased glycolytic activities and a reduced capacity for oxidative phosphorylation. Additionally, SARS-CoV-2 and MERS-CoV infections both presented dysregulated pentose phosphate pathways (PPP), while influenza infection elicited an increase in PPP activity in correlation with disease severity and mortality. Rhinovirus infection, on the other hand, had little effect on the overall perturbation of host cellular metabolism, making it the mildest respiratory infection studied.
The NIMML Institute has an extensive track record of successfully applying advanced computational immunology approaches in precision medicine initially under the Modeling Immunity for Biodefense program. Several seminal papers have been published describing these results. A paper published in Gigascience details the use of agent-based modeling to identify Th17 cells as having a detrimental effect on the gut epithelium during infection by Helicobacter pylori. A study in Scientific Reports identified genes with novel regulatory function through bioinformatics expression pattern analysis of global transcriptomics dataset from a H. pylori co-culture system. Other publications in Inflammatory Bowel Dis. and Scientific Reports describe the development of LANCL2 as a novel therapeutic target for autoimmune disease, including ulcerative colitis and Crohn’s disease. The NIMML Institute recently published a Nature-Systems Biology and Applications article describing the development of a computational model that integrates immunological pathways and key metabolic processes to study immunometabolic mechanisms in CD4+ T cells. These and other legacy computational modeling research and development efforts have been integrated successfully in NIMML’s TITAN-X platform.
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit foundation focused on applying transdisciplinary, team-science approaches to precision medicine. The NIMML Institute applies its TITAN-X advanced A.I. platform to large-scale transdisciplinary projects aimed at solving important public health problems through precision medicine. NIMML combines the expertise of immunologists, computational biologists, toxicologists, computational modelers, translational and clinical researchers, and molecular biologists to translate novel scientific discoveries into medicines for human diseases. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
